
Serraj Andalussi 1*, M. Benhessou1, M.Ennachit 1, M.Elkerroumi 1, A.khoaja 2, S.Benayad 2, N.bennani 2, F.Marnissi 2, M.karkouri 2
1 Mohamed 6 center for cancer treatment1, Ibn Rochd University Hospital Center, Casablanca, Morocco
2Central Pathological Anatomy Service, Ibn Rochd University Hospital Center, Casablanca, Morocco
*Corresponding Author: Serraj Andalussi, Mohamed 6 center for cancer treatment1, Ibn Rochd University Hospital Center, Casablanca, Morocco.
Received date: March 12, 2021
Accepted date: March 25, 2021
Published date: March 29, 2021
Citation: Andalussi S, M Benhessou, M Ennachit, M Elkerroumi, A khoaja, Breast-Localized Granular Cell Tumor Simulating Breast Cancer : About A Case And Review of the Literature. International J of Clinical Gynaecology and Obstetrics, 2(1) ; DOI: http;//doi.org/03.2021/1.1010.
Copyright: © 2021 Serraj Andalussi. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Granular cell tumor (GCT) is a rare, benign tumor that develops from ubiquitous seat Schwann cells; Breast localization is rare. It can be confused with a malignant tumor, clinically and radiologically. Only the histological aspect allows the diagnosis to be made. It is characterized by a proliferation of large cells with abundant granular eosinophilic cytoplasm.
We report an observation of a granular cell tumor of the breast in a 44-year-old woman consulting at the Mohammed IV center for the treatment of cancers at CHU CASABLANCA. The diagnosis was confirmed by pathological examination. The course of the granular cell tumor is often favorable. Surgery remains the treatment of choice. The positive diagnosis is exclusively pathological.
Introduction
The granular cell tumor (GCT) is a benign and ubiquitous lesion preferentially located in the ENT sphere. It was described by Abrikossoff in 1931. Breast localization is rare, presenting only 5 to 15% of all cases (1). It is a puzzling lesion for clinicians and radiologists by its presentation simulating a mammary carcinoma, and for pathologists by its rarity. Its diagnosis is based on the pathological examination[2]. Its prognosis is favorable after complete surgical excision.
We report an observation of a granular cell tumor of the breast by discussing the diagnostic and therapeutic features of this rare tumor.
Observation
A 44-year-old woman consulted for a nodule in the right breast nodule in her right breast that had been developing for nine months, discovered by self-examination. The physical examination found a single infracentimetric nodule of the infero-internal quadrant of the right breast, mobile in relation to the superficial and deep plane without inflammatory signs. The lymph node areas were free. The mammogram did not show any increased opacity or any suspect focus of microcalcifications but just macrocalcification straddling the right internal quadrants.
Breast ultrasound revealed at the level of the right QII a coarsely rounded, hypoechoic formation breaking the connective spans not vascularized on Doppler with posterior attenuation, measuring 9, 1x8.4mm, classified BIRADS IV (Fig. 1).
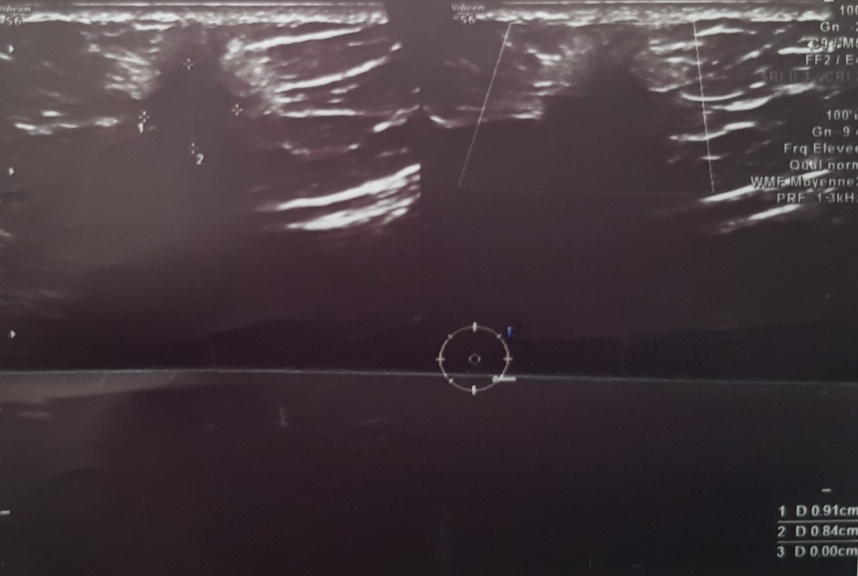
Figure 1 : coarsely rounded formation, hypoechoic breaking the connective tissue with posterior attenuation, measuring 9, 1x8,4mm classified BIRADs IV
The patient had undergone an oriented lumpectomy removing a snow-white, firm, poorly limited nodule 1.5X1.5X1.3cm in diameter with healthy margins.
The extemporaneus examination had concluded in a poorly differentiated and invasive mammary carcinoma measuring 1.5 cm. Consequently, an additional axillary lymph node dissection comprising 10 lymph nodes returned in favor of a reactive adenitis.
Histological cross-sections were performed from the outset. The final examination had shown a tumor proliferation arranged in cords and cell trabeculae, infiltrating the adipose lobules. Tumor cells are large polyhedral with abundant granular eosinophilic cytoplasm (Figure 2).
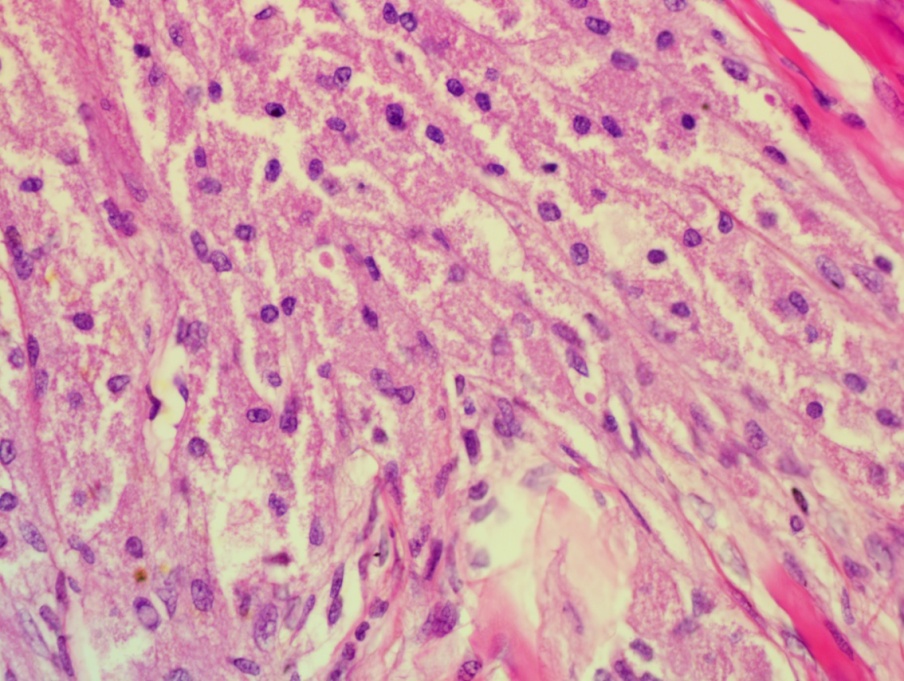
Figure 2:Proliferation made up of large rounded cells with barely visible cytoplasmic boundaries and granular, eosinophilic cytoplasm (HE × 400).
The nuclei are oval or rounded with minimal to moderate atypia. Mitoses are rare and do not exceed 1mitoses / 10CFG. The stroma reaction is hyalinized fibrous; there is no focus of necrosis or spindle cell component seen. The immunohistochemical study showed that these cells intensely express the anti CD68 antibody (figure 3) and do not express the CKAE1 / AE3.
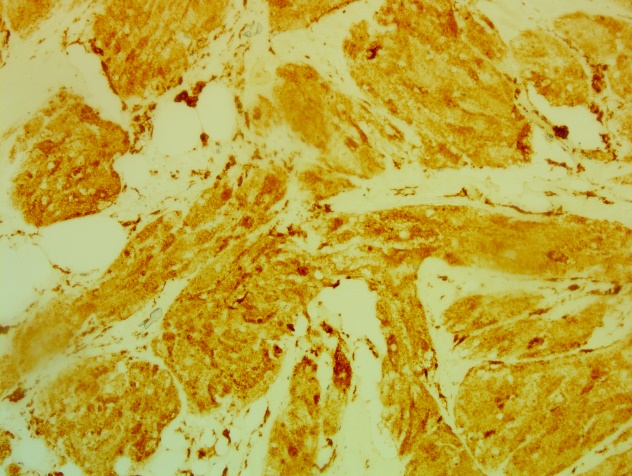
Figure 3: Positive immunostaining for CD68
The complement of immunostaining by the anti-protein S100 (PS100) antibodies (figure 4) shows a diffuse and intense nuclear and cytoplasmic labeling.
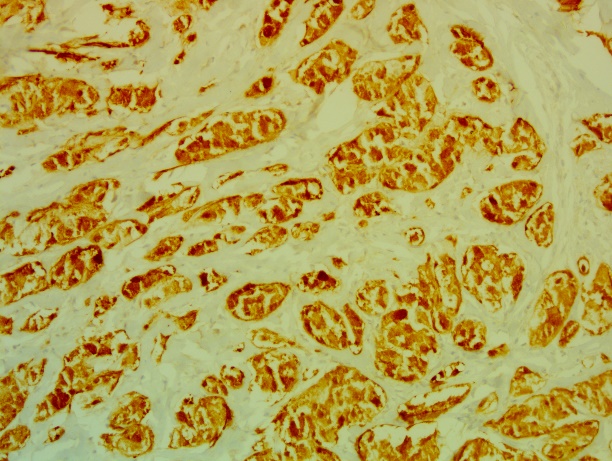
Figure 4: Positive immunostaining for PS100
The diagnosis of benign mammary GCT was retained.
The surgical excision margins were healthy.
The evolution is favorable after four years of surveillance.
Discussion
TCG is a rare entity first described by Weber in 1857 [1]. The main locations are the oral cavity (50% of GCTs) followed by soft tissues [3,4,5]. The other locations are rarer: external genitalia, digestive tract, bladder, breast, salivary glands, nasopharynx [1]. The histogenesis of GCTs remains controversial and their etiology is unknown. The striated muscle origin has been suggested for a long time, hence its name "granular cell myoblastoma" [1]. In 1926, Abrikossoff spoke of his Schwannian origin [6]. Currently, the nervous origin is well established thanks to ultrastructural studies (lysosomal nature of intra cytoplasmic grains) and immunohistochemistry (tumor cells expressing neurogenic markers: PS100 and NSE) [7]. Breast GCT accounts for between 5% and 15% of all GCT cases [7, 8]. It occurs in women between 30 and 50 years old, and especially in women of African American descent. The average age of onset of breast GCT is 38 years. However, some cases of breast GCT have been described in humans [3,4,5].
Clinically, GCT usually presents as a painless, well-circumscribed, and mobile tumor. This tumor usually presents as a painless lump, usually well circumscribed and mobile. The clinical presentation can simulate a malignant tumor (hard mass, poorly limited and fixed in relation to the two superficial and deep planes) [9]. It is usually unique, but multifocalite has been reported in 5.4% to 17.6% of cases [4,5]. The preferred site of these mammary tumors is the upper quadrant, in particular the upper internal quadrant, unlike mammary carcinoma which is most often located at the level of the upper external quadrant. This reflects the course of the supraclavicular nerve. However, GCT showed variable breast locations including the upper outer quadrant, the infero-superior quadrant, the axillary region, the midline, the nipple and the subareolar region [3,8]. In our patient, the tumor was heterogeneous, irregular measuring 2 cm long and located at the level of the upper outer quadrant. The GCT shows a variable non-specific appearance on imaging. Mammographic and echographic presentation is variable [10].
On mammography, it may appear as a well-circumscribed small mass (less than 3cm) or as a poorly defined, spiculated lesion simulating a carcinomatous lesion [4,5]. Ultrasound most often shows a heterogeneous nodule with a posterior shadow cone and irregular contours. More rarely, it shows a hypoechoic nodule with clear limits [2]. The macroscopic appearance presents as a firm or hard lesion, homogeneous, well defined, of greyish white or yellowish color, measuring less than 3cm, but a size of up to 6 cm has been reported. The definitive diagnosis of a GCT is pathological on microbiopsy or ultrasound-guided percutaneous biopsy, thus allowing good sampling before tumor resection to optimize treatment and avoid radical surgery [2]. On histology, the TCG shows a monomorphic tumor proliferation arranged in sheets or nests, it is made of large cells, polygonal or spindle-shaped. The cytoplasm is abundant, granular and eosinophilic. The nuclei are small; round or oval, central, hyperchromatic and regular without atypia or mitotic activity. The stroma is thin and collagenic [11]. Tumor cells show intense postivity to histochemical staining of PAS and PAS diastase [11,12]. In immunohistochemistry, tumor cells are almost always positive for neurogenic markers: S100 protein (100% of cases) and NSE (90% of cases) [13]. which confirms the diagnosis. They are negative for muscle markers (smooth muscle actin and desmin) and epithelial markers (cytokeratins and epithelial membrane antigen) [11,12]. They were also positive for CD68, the carcinoembryonic antigen and vimentin in some cases in the literature [3,12].
The majority of mammary GCTs are benign, the malignancy remains exceptional (less than 1% of breast GCTs) [4,5,11]. The criteria of malignancy are variable. They are proposed by Le et al. [14] and Adeniran et al. [11]. These are necrosis, spindle cells, vesicular nuclei, large nucleolus, mitotic index> 2 / 10fields and nuclear pleomorphism. The presence of two of these six criteria defines atypical GCT and three of these six criteria allows the tumor to be considered as malignant. Surgical treatment is the treatment of choice and consists of wide resection with healthy margins without axillary dissection [15]. The prognosis is often favorable. However, the evolution can be marked by recurrences; in fact, publications have observed recurrences in 2 to 8% after surgical excision despite the achievement of large margins [7, 16]. Note that even in these cases, the prognosis was favorable [12].
Conclusion
GCT is a rare ubiquitous benign neurogenic tumor preferably located on the ENT sphere. The mammary location is rarely difficult to diagnose clinically and radiologically. It can be confused with an infiltrating malignant tumor on mammography and ultrasound. The diagnosis with certainty cannot be certain established only on immunohistological analysis of the complete lesion. Its treatment is surgical. Its evolution is often favorable, however, clinical and radiological monitoring of resected mammary GCTs is recommended. This observation illustrates the rarity of mammary GCT, which any pathologist should think about in the face of benign and malignant lesions in order to avoid an erroneous diagnosis which could lead to unnecessary surgery.
Declaration of interests
The authors declare that they have no conflicts of interest in relation to this article.
Contributions from authors
All authors have read and approved the final version of the manuscript.